Revisited: A Guide to Reverse Osmosis

What is Osmosis?
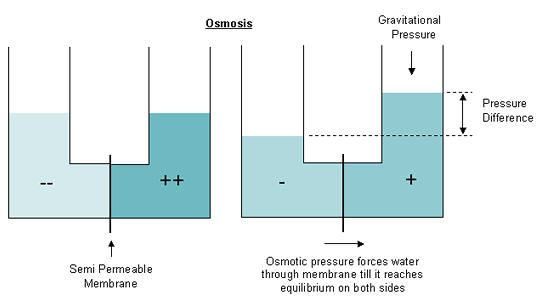
Osmosis is the diffusion of water through a semi-permeable membrane between two liquids of different salt concentrations. By nature, the two solutions will want to reach a state of equilibrium with each other. The semi-permeable membrane restricts salts and most other dissolved solids, allows very pure water to pass from the less concentrated side in an effort to dilute the more concentrated side. Equilibrium is achieved once the atmospheric pressure differential between the two liquids is equal to the osmotic pressure at the membrane.
In the diagram below the (--) side of the membrane represents a solution with relatively low salinity and the (++) side represents a higher concentration. In the first picture the water levels are equal, but with time, water from the (--) side will slowly diffuse through the membrane and decrease the salt concentration on the (++) side. The second picture shows how the diffusion of water through the membrane reduced the water level on the less concentrated side (-) and increased the level of water on the more concentrated side (+). The differences in water levels create an increased gravitational pressure opposing the osmotic pressure at the membrane. If this pressure reaches the level of osmotic pressure before there is equilibrium between the two solutions then the diffusion will cease.
How does reverse osmosis work?
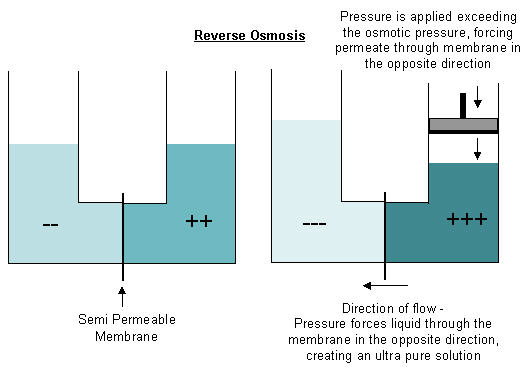
Reverse osmosis is achieved by forcing the direction of the diffusion of liquid through the membrane in the opposite direction. In order to reverse the flow of fluids a pressure exceeding the natural osmotic pressure at the membrane must be applied on the concentrated side of the membrane. This process forces water through the membrane creating a high purity, or permeate, stream on one side of the membrane and a solution of increased concentration on the opposite side of the membrane. This process uses the unique properties of the membrane to create a highly pure solution.
In the picture below, we again have two solutions of different salinities separated by a semi-permeable membrane. In order to have water flow in the opposite direction, a pressure must be exerted on the more concentrated (++) side. This pressure must exceed the osmotic pressure at the membrane, as water from the less concentrated side(--) will naturally attempt to diffuse through the membrane and dilute the concentrated solution. Once sufficient pressure is applied, the permeate is forced through the membrane, further diluting the less concentrated side (--) and making the concentrated solution (++) even more concentrated. How does reverse osmosis work?
How does Reverse Osmosis benefit aquarists?
Reverse osmosis is the most economically efficient way to derive near pure water from a source with higher levels of dissolved solids. The process is used to create most of the non-spring bottled water available at grocery stores today and for deriving freshwater from saltwater and brackish water sources. Reverse osmosis has also gained tremendous popularity with aquarists in recent years. Using purer water allows the aquarist to have greater control over what chemicals are put into the tank water. This eliminates the need for the aquarist to treat tap water for excessive levels of chlorine, trace metals, phosphates, nitrates and more. Reverse Osmosis systems are relatively affordable ranging between $100-200 for a mid-level system.
What are all of those other stages seen on most consumer units used for?
The other stages are used to condition the water and remove as many impurities as possible from the source water before entering the chamber with the osmotic membrane. Such chambers usually include but are not limited to:
- Mechanical filter Typically a spiral bound felt material designed to trap silts and suspended solids, permitting only very small particles to pass, usually in the 10-100 micron range.
- Carbon filter The pre-filtered water is then usually sent through a chamber with a carbon filter to remove chlorines and other oxidizers, otherwise oxidizers can severely reduce membrane efficiency and sometimes even cause damage.
- Water Softener Another possible but more uncommon stage used in consumer units is a water softener. This will reduce the water hardness and prevent excessive scaling or build up of solids (calcium and magnesium ions which precipitate out of solution as waste water TDS rises) across the membrane. Using water softeners can increase the efficiency and prolong the useable life of the membrane.
- De-Ionization Resin In RO/DI systems after passing through the osmotic membrane, the effluent is sent to another chamber for de-ionization (DI). The cartridge contains a mixed bed of anion and cation resins which use an ion exchange mechanism to remove impurities that can pass through the RO membrane. For example, Silica, phosphates, nitrates, ammonium and other trace impurities may not be completely removed before this point.
Other options to further improve efficiencies:
- Booster pumps increases the pressure of the feed water to the RO unit. The increase in pressure allows a greater efficiency of the osmotic membrane and reduces the amount of waste water.
- Flush valve this is a simple valve which is placed between the membrane reject port and the flow restrictor. It provides a high flow by-pass to purge concentrated waste water from the membrane housing, and flush particulates from the membrane surface. This very inexpensive feature can significantly increase the useful life of the membrane, especially when utilized after each use.
- Piggy back kits these kits incorporate an additional RO membrane into an existing system and can some times more than double the production of permeate water and further reduce the amount of waste water going to the drain.
How can reverse osmosis benefit fish holding facilities and retail stores?
Retail stores and holding facilities can also use the benefits of pure water for their own use. Larger commercial units offer a more efficient version of the units commonly available to aquarists. These units can produce a far greater amount of product water and have much lower percentages of waste water. Cost of operation is relatively inexpensive and the units only require moderate maintenance.
Commercial units typically employ a larger system of pre-filters to adequately treat all feed water prior to entering the membrane chamber. These pre-filters should be chosen specifically to suit your water supply, as the chemical makeup of municipal water varies from location to location. Commercial units also utilize a large pressure pump in order to provide a large pressure forcing the permeate through the membrane.
Using purified water can increase water quality and can help to improve survivability rates of fish and coral. Some types of coral are very sensitive to higher levels of phosphates and silicates. Acropora species, among other types of hard coral, can turn brown and possibly undergo tissue necrosis with elevated levels of these chemicals.
Stores can also use larger units to sell RO purified water to customers who dont have RO units. Special promotions where customers can purchase buy in advance cards with discounts on larger amounts purchased are good ways to market the water to customers. Stores can also combine promotions for selling synthetic salts with a specific amount of RO purified water.